Did you know that there are two definitions of time and they do not agree!
In our daily lives, most of us experience time as a certainty, always moving in the right direction, at a rate that can be easily measured and agreed upon by all observers. But when two observers compare what they are experiencing, for themselves, as one second, they do not always agree. This was not explained until the early twentieth century, with the emergence of Einstein's theory of relativity. The surprise is that the same time, long considered fundamental and universal, is actually relative, meaning that different observers will feel the flow of time differently from each other, as long as they move through space at different speeds or in different directions. Whether two events occur simultaneously or one occurs before the other, depends entirely on the observer's point of view.
Recommend
Show key points
- Time is traditionally perceived as a consistent and measurable phenomenon, but Einstein's theory of relativity revealed that it is actually experienced differently depending on the observer's motion and perspective.
- Although time feels subjective and relative among observers, everyone still experiences it as moving steadily forward—this consistent forward flow is known as the arrow of time.
- The laws of physics, from Newtonian mechanics to Einstein’s equations, do not inherently prefer a specific direction of time, making the unidirectional flow of time a puzzling phenomenon.
- ADVERTISEMENT
- Entropy, a measure of disorder or the number of possible arrangements in a system, is thought to be closely linked to our experience of time's forward motion.
- The second law of thermodynamics states that entropy in a closed and isolated system can only increase or stay constant, implying a built-in direction to time in such systems.
- In everyday life, we observe irreversible processes—like melting ice or mixing coffee—that increase entropy, reinforcing the perception of time moving only forward.
- While reversing entropy in a system is theoretically imaginable, in practice it is virtually impossible due to the constraints imposed by thermodynamic laws.
Time arrow, or time arrow:

But, despite the ambiguity of time, there are some facts about it that all observers can agree on. Perhaps the most fundamental of these facts, and perhaps the most confusing, is that everyone, in his idle reference sentence, sees time always moving forward at the same rate: one second every second. This fact is known as the "arrow of time", or specifically our perceptual time arrow. There are many ideas about what makes us experience it the way we experience it, and one idea that has been put forward is the only other "arrow of time" that we know: the arrow of time defined by thermodynamics.
Single side:

With every passing moment, no matter what happens around us, we find ourselves experiencing the most primitive and monotonous form of time travel: the slow passage of time that passes us as we progress into the future. With each passing moment, time continues to propagate to the direction where it was moving, maintaining its constant speed, moving the right distance at each given time interval, regardless of what is happening around it. At any moment, in any circumstance, time does not seem to hold or reverse; it can only continue to advance into the future. In other words, the arrow of time always points to the forward direction of anything inside our universe. But this is a mystery to fundamental physics, as there is no explanation for why it behaved in this way. The laws of nature, with very few exceptions, are completely chronologically symmetrical. From Newton to Einstein, the equations that govern reality have no preferred direction for the flow of time. The behavior of any sentence can be described by equations that work in the forward direction as well as in the reverse direction. For example, we can move "backward" as well as "forward" in any of our three spatial dimensions. But somehow, time is different.
Entropy:
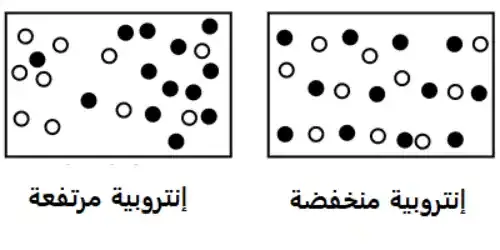
With all this in mind, where does our arrow of time come from? According to many people, there seems to be a proposed link between what we perceive as the arrow of time and a quantity called entropy, commonly known as the "scale of chaos" in a physical sentence. If you had more options for how to arrange the sentence so that it remains the same, you had higher entropy than if there were fewer options. For example, a box with a "hot" and "cold" side separated by a barrier has less entropy than a box in which the elements mix well after the barrier has been removed.
The relationship between time and entropy:

Whenever we discuss entropy, we need to keep in mind that we are constrained by the laws of thermodynamics. In particular, the second law is of great importance, as it states that the entropy of closed and isolated sentences that do not allow the exchange of matter or energy with the external environment can only increase or remain the same over time; Since the universe is closed and almost isolated (this approximation is very good for almost all applications), the entropy of the entire universe must increase over time. It is the only known law in physics that seems to show a preferred direction of time.
Does this mean that it is possible to test time the way we do it only because of the second law of thermodynamics? If so, this suggests a deep connection between the arrow of time and entropy. If the arrow of imagined time is always moving forward, no matter what happens to entropy within a sentence, then this proposed correlation would not be real. But reversing the flow of entropy inside most sentences is easier said than done; we can easily beat an egg and then cook it, but vice versa returning the egg after cooking it to its initial position and separating its components is not as easy. The same situation applies when we dissolve sugar and cream in coffee; homogenizing the mixture is much easier than separating its ingredients. In both examples, the entropy in the final state is higher than in the initial state. So, in practice, entropy reversal never occurs automatically. This may determine the thermodynamic arrow's direction forward as entropy increases.
Nature is full of examples such as mixing coffee with cream or whipping and cooking eggs: what we traditionally call "irreversible reactions" in physics. If you drop an ice cube into warm juice, the ice will melt, resulting in a cold juice at a uniform temperature below its temperature before the ice cube was placed in it. But it is impossible for a cooled juice to automatically separate into warm juice and an ice cube; this is prohibited under the second law of thermodynamics. This is the price that thermodynamic laws draw from the universe over time: the total entropy of a closed and isolated system can never decline.
![]()
10 things smart people never share with anyone
Smart people often keep quiet about their true knowledge, finances, personal struggles, and future plans. It’s not secrecy—it’s strategy. By choosing what to share, they protect their peace, maintain control, and keep life’s noise at bay. more- ADVERTISEMENT
![]()
5 electronic games to develop your skills and intelligence
Discover how video games like SimCity, Stardew Valley, and Gorogoa can sharpen your mind, boost creativity, and help with planning and decision-making—all while having fun. Not all games are just for play; some are powerful tools for personal growth and learning. more- ADVERTISEMENT
![]()
Marseille: the city where everyone belongs to another place
Marseille is a vibrant coastal city where cultures from around the world blend in daily life. From Arabic to French and Italian, its streets echo with languages and stories of migration, making it a unique place filled with diverse food, traditions, and peaceful coexistence among its residents. more- ADVERTISEMENT
![]()
LI-FI: where the Internet travels at the speed of light
LI-FI, created in 2011 by Prof. Harald Haas in Scotland, uses light instead of radio waves for data transfer. It offers ultra-fast speed and strong security, making it ideal for sensitive environments. Despite its promise, challenges like high costs and dependency on constant lighting limit its widespread use. more- ADVERTISEMENT
![]()
Will the earth keep heating up?
The summer of 2023 brought record heat, fierce wildfires, and deadly monsoons—clear signs of worsening climate change. Scientists warn we’re edging close to the 1.5°C global warming limit, urging urgent action. Real change means cutting emissions now and rethinking our everyday choices—are we ready to act? more- ADVERTISEMENT
![]()
How to overcome your fear of flying
How to overcome your fear of flying more- ADVERTISEMENT
![]()
Success Guide - How to discover your passion?
Discovering your passion is a personal journey that brings meaning and joy to life. It’s about finding what excites you, motivates you, and makes you feel alive. With patience, reflection, and trying new experiences, you can uncover what truly inspires you without needing to spend a fortune. more- ADVERTISEMENT
![]()
Best castles worth visiting near Edinburgh
Edinburgh is a castle lover's dream, with iconic sites like Edinburgh Castle atop Castle Rock and the mysterious Crichton Castle with Game of Thrones vibes. From romantic Rosslyn to tranquil Lauriston, each castle tells a unique story, making the city’s history come alive in the most unforgettable way. more- ADVERTISEMENT
![]()
Dar Al-Hajar ... The seven-story stone palace in Wadi Dhahr, Yemen
Perched atop a massive rock near Sana'a, the Stone Palace blends seamlessly into the mountain, appearing ancient though built in the 1930s. Once a royal summer retreat, it's now a museum showcasing Yemen’s rich architecture, with its war-castle design, gardens, and maze-like interior captivating all who visit—or admire from afar. more- ADVERTISEMENT
![]()
The concept of financial liberalization - how can you achieve it?
The Financial Freedom Concept - How can you achieve it more- ADVERTISEMENT